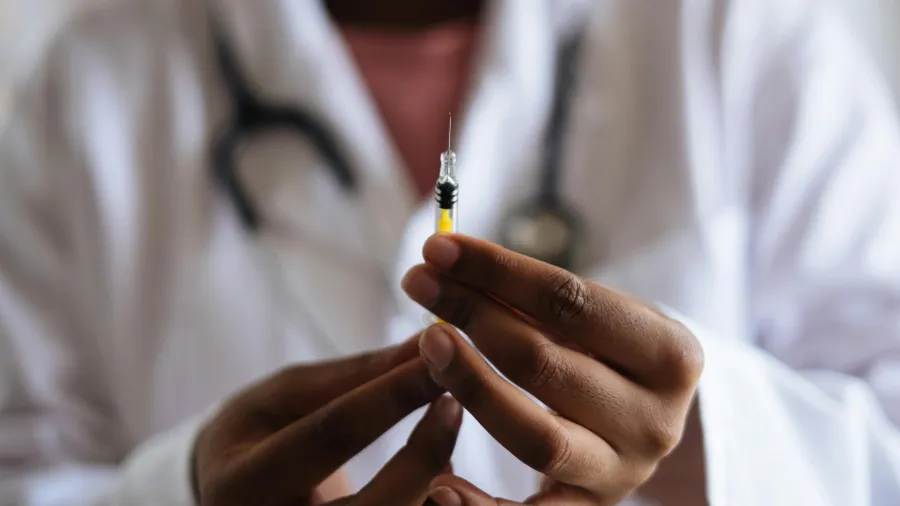
Gov’t OKs Pfizer’s dual indication vaccine vs. respiratory syncytial virus
This is the first bivalent, single-dose vaccine against RSV.
Pfizer Inc. said it has secured marketing authorization from the Department of Health for the company’s bivalent RSV prefusion F vaccine to fight lower respiratory tract disease (LRTD) and severe LRTD caused by the respiratory syncytial virus (RSV).
The company noted this is the first and only bivalent, single-dose vaccine that fights both the RSV A and RSV B disease. It is also the only vaccine shot suitable for individuals aged 60 years and older, and infants from birth up to six months of age through the active immunisation of pregnant individuals.
Pfizer explained that the virus can affect the lungs and breathing passages of an infected individual, potentially causing serious complications or death in babies.
In 2019, 33 million cases of RSV-associated acute lower respiratory infection were recorded globally, with 101,400 deaths in children aged 5 years and younger.
RSV is the leading viral cause of hospitalisation in Hong Kong due to common respiratory viruses in children under the age of one.
Two clinical trials were conducted to test the vaccine, which involved older adults, and newborns and young infants.
“This vaccine can help babies and older adults in Hong Kong fight against RSV-associated LRTD, and we are working towards making it available to the market as soon as possible,” said Krishnamoorthy Sundaresan, Pfizer’s Hong Kong and Macau market lead.
ALSO READ: CUHK develops tool for early hypoglycemia warnings amongst high-risk adults
The vaccine is expected to be available in Macau by June 2024, then in Hong Kong by August 2024.






![Cross Domain [Manu + SBR + ABF + ABR + FMCG + HBR + ]](https://cmg-qa.s3.ap-southeast-1.amazonaws.com/s3fs-public/styles/exclusive_featured_article/public/2025-01/earth-3537401_1920_4.jpg.webp?itok=WaRpTJwE)









 Advertise
Advertise


